Menu
Add Your Heading Text Here
About Malaria
Index
About Malaria
Malaria is a life-threatening disease caused by protozoan parasites of the genus Plasmodium. The disease is transmitted to humans through the bite of infected female Anopheles mosquitoes. There are five species of Plasmodium that commonly infect humans: Plasmodium falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi. Among these, P. falciparum is the most deadly, responsible for the majority of severe cases and deaths, particularly in sub-Saharan Africa. P. vivax is the most widespread and can cause relapsing malaria due to dormant liver stages called hypnozoites. The life cycle of Plasmodium involves both human and mosquito hosts, with complex stages including liver infection (exoerythrocytic) and red blood cell infection (erythrocytic).
Burden
Malaria remains one of the world’s most pressing public health issues.
As of 2024, malaria continues to pose a significant global health challenge, with the disease remaining prevalent, particularly in low-income regions. In 2022, an estimated 249 million cases of malaria were reported globally, resulting in approximately 608,000 deaths. The burden of the disease is heavily concentrated in the WHO African Region, which accounted for 94% of all cases and 95% of deaths. This region remains disproportionately affected, with young children and pregnant women among the most vulnerable groups.
Children under five years of age are particularly at risk, comprising 78% of malaria-related deaths in the African Region. The disease’s prevalence in these areas is exacerbated by challenges such as limited access to healthcare, inadequate vector control measures, and socioeconomic factors.
Despite ongoing efforts to control and eliminate malaria, progress has been uneven. Factors like resistance to antimalarial drugs and insecticides, as well as environmental changes, complicate eradication efforts. The economic impact of malaria is also substantial, affecting the productivity and development of affected countries.
Global initiatives continue to focus on improving preventive measures, enhancing access to treatment, and addressing the socioeconomic determinants of health that contribute to the persistence of malaria. These efforts are crucial in striving toward a more equitable world where the burden of malaria is significantly reduced.
History
Ancient History
Malaria has afflicted humans for millennia, with its presence documented in various ancient civilizations. The disease’s impact on history is profound, influencing not only the health but also the culture, economy, and warfare of ancient societies.
In Ancient Egypt, references to malaria-like symptoms are found in medical texts such as the Ebers Papyrus (circa 1550 BCE), one of the oldest medical documents. The Egyptians attributed the disease to the influence of evil spirits and sought to treat it with various herbal remedies. The condition was described as “heating of the head,” indicative of fever, a common symptom of malaria.
Chinese records also provide early descriptions of malaria. The Nei Ching (The Canon of Medicine), attributed to the Yellow Emperor and dating back to 2700 BCE, mentions intermittent fevers. This ancient medical text outlines treatments using qinghao (Artemisia annua), a plant later identified as a source of artemisinin, a key compound in modern antimalarial drugs.
In Mesopotamian civilization, cuneiform tablets from as early as 2000 BCE contain descriptions of ailments characterized by chills and fever, which are consistent with malaria symptoms. The Assyrians and Babylonians believed these fevers were caused by demons and sought cures through prayers and rituals.
Malaria was also well-known in Ancient Greece, where it significantly impacted the health and lives of the population. The Greek physician Hippocrates (460–370 BCE) documented the symptoms of malaria, distinguishing between the different forms of fever patterns, such as tertian (fever every third day) and quartan (fever every fourth day), which correspond to infections caused by Plasmodium vivax and Plasmodium malariae, respectively.
In Rome, malaria had a profound impact on the city’s development. The disease was rampant in the marshes around Rome, which were later known as the Pontine Marshes. Malaria was referred to as “Roman fever,” and its prevalence was thought to contribute to the decline of the Roman Empire by debilitating the population and military. The term “malaria” itself comes from the Italian words “mala aria,” meaning “bad air,” a reference to the mistaken belief that the disease was caused by foul air from swamps.
Malaria was also present in other ancient civilizations, including the Indian subcontinent, where it was described in Ayurvedic texts. The Sushruta Samhita, an ancient Sanskrit text, mentions the symptoms of intermittent fever, known locally as “vishama jwara.”
Scientific Discoveries
The history of malaria research is marked by several key scientific discoveries that have significantly advanced our understanding of the disease and its management. Here are some of the most important milestones:
Discovery of the Malaria Parasite (Plasmodium)
- The first major breakthrough in understanding malaria came when Charles Louis Alphonse Laveran (1880), a French military doctor, observed parasites in the blood of a malaria patient. During his observations of blood samples from patients suffering from malaria, he noticed peculiar structures within the red blood cells. He identified these as pigmented granules, which he later associated with the presence of a microorganism. Through meticulous observation, Laveran noticed that these structures were not static. He observed the movement of flagella in some of the cells, which indicated a living organism. He identified this as a new type of microorganism and proposed that it was the cause of malaria. Laveran identified these microorganisms as protozoa, naming them Plasmodium. This discovery was pivotal as it was the first time a protozoan was recognized as a cause of human disease, and earned Laveran the Nobel Prize in Physiology or Medicine in 1907. Subsequent research by other scientists, such as Ettore Marchiafava and Angelo Celli, corroborated Laveran’s findings and expanded the understanding of the malaria parasite, identifying different species responsible for various forms of the disease.
- Role of Mosquitoes in Transmission
- Sir Ronald Ross (1897), a British medical officer, discovered that Anopheles mosquitoes transmit malaria. He observed malaria parasites in the stomach of these mosquitoes after they fed on infected patients, thus proving the mosquito’s role in the parasite’s life cycle. In 1898, Ross used birds infected with the avian malaria parasite Plasmodium relictum to further his studies. He showed that mosquitoes could acquire the parasite from infected birds and then transmit it to healthy birds, proving the vectorial capacity of mosquitoes. This work earned him the Nobel Prize in 1902. Giovanni Battista Grassi (1898), an Italian scientist, further confirmed that Anopheles mosquitoes were the specific vector for human malaria, detailing the life cycle stages in both mosquitoes and humans. In 1898, almost concurrently with Ross, Grassi and his colleagues Amico Bignami and Giuseppe Bastianelli collected Anopheles claviger mosquitoes and allowed them to feed on malaria-infected patients. They then observed the development of the parasite within the mosquitoes, confirming that sporozoites formed in the salivary glands were capable of transmitting the disease to humans. In a landmark experiment, they allowed infected mosquitoes to bite healthy volunteers, who subsequently developed malaria, providing direct evidence that mosquitoes were not just carriers of the parasite but were essential for the disease’s transmission to humans.
Discovery of the Liver Stage of Infection
- Henry Shortt and Cyril Garnham (1948) demonstrated that after Plasmodium sporozoites enter the bloodstream, they travel to the liver where they undergo an asymptomatic phase of development. Prior to the 1940s, it was known that Plasmodium parasites caused malaria by infecting red blood cells, leading to the symptoms associated with the disease. Researchers suspected that there might be a phase in another organ before the parasites entered the bloodstream, but no definitive evidence had been found. Shortt and Garnham conducted a series of experiments using the rhesus monkey (Macaca mulatta), which they infected with sporozoites from the mosquito Anopheles stephensi carrying Plasmodium cynomolgi. Through careful and systematic examination of the monkeys’ liver tissues at various time points after sporozoite inoculation, Shortt and Garnham observed a new phase of the parasite’s lifecycle within their liver cells. They termed these parasite forms “exoerythrocytic stages”, as they represented a previously unknown developmental stage occurring outside of red blood cells. These exoerythrocytic forms were seen as large, spherical bodies in the liver cells, containing thousands of merozoites. The identification of the liver stage, or pre-erythrocytic phase, of Plasmodium infection was a monumental advancement in the understanding of malaria, and explained why there was a delay between being bitten by an infected mosquito and the onset of symptoms.
Elucidation of the Full Life Cycle of Plasmodium
The life cycle of Plasmodium parasites involves several stages that take place in two different hosts:
- Sporozoite Stage: Infective sporozoites are injected into the human bloodstream by an infected Anopheles mosquito during a blood meal.
- Liver Stage: Sporozoites travel to the liver, where they invade hepatocytes and develop into schizonts inside a parasitophorous vacuole, eventually releasing merozoites into the bloodstream. P. vivax and P. ovale can form hypnozoites, dormant liver forms that can reactivate and cause relapses.
- Blood Stage: Merozoites invade red blood cells, where they multiply asexually. This stage is responsible for the clinical symptoms of malaria.
- Sexual Stage (Gametocytes): Some blood stages differentiate into sexual forms called gametocytes, which are taken up by mosquitoes during a blood meal.
- Mosquito Stage: In the mosquito gut, gametocytes mature into gametes, fuse to form a zygote, which develops into an ookinete, penetrates the gut wall, and forms an oocyst. The oocyst produces sporozoites, which migrate to the mosquito’s salivary glands, completing the cycle.
Symptoms
Malaria symptoms can range from mild to severe, depending on several factors such as the Plasmodium species involved, the individual’s immune status, and access to timely treatment. Chronic malaria occurs when a person remains infected with malaria parasites for an extended period, often lasting months or even years.
Mild malaria
- Symptoms of mild malaria include fever, shivering, arthralgia (joint pain), vomiting, anemia (caused by hemolysis), hemoglobinuria, retinal damage, and convulsions. The classic symptom of malaria is cyclical occurrence of sudden coldness followed by rigor and then fever and sweating lasting four to six hours, occurring every two days in P. vivax and P. ovale infections, while every three for P. malariae. P. falciparum can have recurrent fever every 36–48 hours or a less pronounced and almost continuous fever.
Severe malaria
- Severe malaria, also known as complicated malaria, occurs when the infection progresses to a stage that poses significant risks to the patient’s health and is often life-threatening. Severe malaria is most commonly caused by Plasmodium falciparum, usually arising 6–14 days after infection, but can occasionally result from other Plasmodium species. Consequences of severe malaria include coma and death if untreated—young children and pregnant women are especially vulnerable. Malaria has been found to cause cognitive impairments, especially in children, as a result of cerebral malaria, to which children are more vulnerable. Cerebral malaria is associated with retinal whitening, which may be a useful clinical sign in distinguishing malaria from other causes of fever. Splenomegaly (enlarged spleen), severe anemia, respiratory distress, metabolic acidosis, jaundice, severe headache, cerebral ischemia, hepatomegaly (enlarged liver), hypoglycemia, hemoglobinuria, acute kidney failure, and shock may occur. Renal failure may cause blackwater fever, where hemoglobin from lysed red blood cells leaks into the urine. Severe malaria can progress extremely rapidly and cause death within hours or days. In endemic areas, treatment is often less satisfactory and the overall fatality rate for all cases of malaria can be as high as one in ten. Over the longer term, developmental impairments have been documented in children who have suffered episodes of severe malaria.
Chronic malaria
- Chronic malaria is characterized by long-term infection with malaria parasites, often with low levels of parasites present in the blood. This condition can occur due to various factors, including partial immunity, the presence of dormant liver stages, inadequate treatment, ongoing exposure to the parasite, and immune system challenges. Unlike acute malaria, which presents with severe symptoms shortly after infection, chronic malaria is characterized by low-grade, often asymptomatic parasitemia that can occasionally or intermittently flare into more noticeable symptoms. In the case of hypnozoite-forming parasites, P. vivax and P. ovale, the disease can relapse months or years after exposure, due to the presence of latent parasites in the liver. The longest incubation period reported for a P. vivax infection is 30 years. Chronic carriers can act as reservoirs for malaria transmission, posing a challenge to malaria elimination efforts. Thus, addressing chronic malaria is critical for eradication initiatives, as undetected and untreated cases can undermine control efforts.
WHO Malaria Eradication Programme (1955-1969)
The malaria eradication campaigns of the 1950s and 1960s were part of a global effort aimed at reducing and ultimately eliminating malaria, a deadly and widespread infectious disease caused by Plasmodium parasites transmitted by Anopheles mosquitoes. These campaigns were driven by advancements in medical knowledge, public health strategies, and international cooperation.
Key Elements of the Campaigns
WHO Global Malaria Eradication Programme (1955-1969):
- Launch: The World Health Organization (WHO) officially launched the Global Malaria Eradication Programme (GMEP) in 1955. The initiative aimed to eradicate malaria worldwide, building on the success of pilot projects in regions such as Sardinia and Greece.
- Strategies:
- DDT Spraying: Widespread indoor residual spraying of houses with the insecticide DDT was a cornerstone of the program. DDT was highly effective in killing mosquitoes and had been used successfully in earlier control efforts.
- Antimalarial Drugs: Chloroquine and other antimalarial drugs were administered to treat and prevent malaria, reducing the parasite load in the human population.
- Surveillance and Treatment: Identifying and treating malaria cases was crucial, along with monitoring mosquito populations and their resistance to insecticides.
Regional and National Efforts:
- Countries and regions participated in the program with varying degrees of success, depending on factors such as political will, financial resources, and the ecological and epidemiological characteristics of malaria in different areas.
- Some regions, particularly in Europe, North America, and parts of the Caribbean, were able to eliminate malaria entirely, while others, like sub-Saharan Africa and Southeast Asia, faced greater challenges.
Outcomes and Challenges
Successes:
- The program achieved notable successes in many regions. For example, by the mid-1960s, malaria had been eliminated from large areas of Europe and North America, and significantly reduced in parts of Asia and Latin America.
Challenges and Limitations:
- Insecticide and Drug Resistance: Over time, mosquitoes developed resistance to DDT, and Plasmodium parasites became resistant to drugs like chloroquine, diminishing the effectiveness of these tools.
- Operational Difficulties: In many developing regions, logistical challenges, such as reaching remote populations, political instability, and inadequate infrastructure, hampered eradication efforts.
- Economic and Political Factors: Sustaining the financial and political commitment required for long-term eradication proved difficult. As a result, resources and efforts waned, particularly as global priorities shifted.
Program Conclusion and Legacy:
- The WHO declared the end of the GMEP in 1969, recognizing that complete eradication was not feasible in the near term. The focus shifted from eradication to control, aiming to reduce morbidity and mortality.
- Despite not achieving global eradication, the campaign led to significant reductions in malaria cases and deaths in many regions and laid the groundwork for future malaria control and elimination efforts.
Impact and Lessons Learned
The malaria eradication campaigns of the 1950s and 1960s provided valuable lessons in public health, particularly in the importance of sustained political and financial commitment, the challenges of developing resistance, and the need for adaptable and comprehensive strategies. These lessons continue to inform current global efforts to combat malaria, with a renewed focus on integrated approaches that include vector control, effective treatment, vaccine development, and robust health systems.
Malaria Control Strategies
Malaria control measures encompass a variety of strategies aimed at reducing the transmission of the disease, managing cases, and ultimately aiming for eradication. These measures are typically categorized into vector control, chemoprevention, case management, and surveillance and response systems. Comprehensive malaria control requires an integrated approach, combining preventive measures, accurate diagnosis, effective treatment, and robust surveillance systems. Continued innovation, funding, and international cooperation are crucial to sustain progress and work towards the global goal of malaria elimination.
Vector Control
- Insecticide-Treated Nets (ITNs) are one of the most effective tools in preventing malaria transmission. These nets are treated with insecticides that not only protect individuals from mosquito bites during sleep but also kill mosquitoes that come into contact with them. Widespread distribution of ITNs, particularly to vulnerable populations such as children under five and pregnant women, has significantly reduced malaria incidence and mortality in many endemic regions.
- Indoor Residual Spraying (IRS) involves spraying the interior walls of homes with long-lasting insecticides. This method kills mosquitoes that rest on the walls, thereby reducing the mosquito population and interrupting malaria transmission. The effectiveness of IRS depends on high coverage and the timely application of insecticides before the peak transmission season.
- Larval Source Management targets the aquatic stages of mosquitoes, reducing the mosquito population by eliminating or treating breeding sites. Methods include environmental management (draining stagnant water), larviciding (applying insecticides to water bodies), and biological control (introducing natural predators of mosquito larvae).
Chemoprevention
- Intermittent Preventive Treatment (IPT) is the administration of antimalarial drugs at regular intervals to vulnerable populations, such as pregnant women (IPTp) and infants (IPTi), regardless of whether they are infected. This strategy aims to reduce the risk of developing severe malaria and related complications by clearing existing infections and providing a protective effect during the highest-risk periods.
- Seasonal Malaria Chemoprevention (SMC) involves the administration of full treatment courses of antimalarial drugs at regular intervals during the high transmission season. It is primarily used in regions with highly seasonal malaria transmission, particularly in the Sahel region of Africa, to protect children under five years of age from infection during peak transmission periods.
Case Management
- Rapid Diagnostic Tests (RDTs) and Microscopy provide accurate and timely malaria diagnosis, which is crucial for effective treatment. RDTs allow for quick detection of malaria infections, especially in remote areas without access to laboratory facilities. Microscopy, though more resource-intensive, provides detailed information on the type and density of parasites. Correct diagnosis ensures that patients receive the appropriate treatment, reducing the risk of severe illness and death.
- Artemisinin-Based Combination Therapies (ACTs) are the recommended first-line treatment for uncomplicated Plasmodium falciparum malaria. They combine artemisinin, which rapidly reduces the parasite load, with a partner drug that eliminates the remaining parasites. This combination reduces the likelihood of drug resistance developing.
Surveillance and Response
- Effective Malaria Surveillance Systems are essential for monitoring malaria cases and guiding control measures. They help in identifying outbreaks, tracking trends in malaria incidence, and assessing the impact of control interventions. High-quality surveillance data are critical for targeting resources, evaluating the effectiveness of interventions, and adapting strategies as needed.
- Rapid Response to Malaria Outbreaks involves identifying and treating cases, distributing preventive measures such as ITNs and IRS, and conducting public health education campaigns. This approach helps to quickly control and contain outbreaks, preventing wider transmission.
Vaccination
- The RTS,S/AS01 (Mosquirix) and R21/Matrix-M Vaccines, approved by the World Health Organization in 2021 and 2023, respectively, provide partial protection against Plasmodium falciparum malaria, particularly in children. While not fully protective, the vaccines reduce the incidence of malaria and severe malaria cases in vaccinated individuals, representing additional tools in the malaria control arsenal.
Public Health Education and Community Engagement
- Community engagement and public health education are vital for the success of malaria control measures. Educating communities about the importance of using ITNs, seeking timely treatment, and participating in IRS campaigns enhances compliance and effectiveness. Community health workers often play a crucial role in disseminating information and supporting malaria control activities at the grassroots level.
Diagnostics
The landscape of malaria diagnostics has evolved significantly, from traditional microscopy to advanced molecular techniques and emerging technologies. Each diagnostic tool has its advantages and limitations, making them suitable for different contexts. While microscopy remains a standard method due to its high specificity and ability to estimate parasite density, RDTs offer rapid and user-friendly options for field settings. PCR and LAMP provide high sensitivity and accuracy, particularly for low parasitemia and species identification, but require more complex infrastructure. Emerging technologies, including nanotechnology and AI, hold promise for future advancements in malaria diagnostics. The continued development and integration of these tools are crucial for effective malaria control and eradication efforts. Malaria diagnistic tools include:
Microscopy
- Description:
- Method: Microscopy is the gold standard for malaria diagnosis. It involves examining a blood smear under a microscope to identify malaria parasites.
- Types of Smears: Thin smears (for species identification) and thick smears (for parasite density).
- Advantages:
- High Specificity: Highly effective in identifying the species of malaria parasites.
- Quantitative Data: Allows for the estimation of parasite density, which is useful for assessing severity.
- Limitations:
- Labor-Intensive: Requires skilled personnel and significant training.
- Sensitivity Issues: Less effective at detecting low parasite densities and in low-resource settings where equipment and training may be limited.
- Recent Developments:
- Digital Microscopy: Advances in digital imaging and artificial intelligence are improving the accuracy and speed of microscopy.
- Description:
- Rapid Diagnostic Tests (RDTs)
- Description:
- Method: RDTs are immunochromatographic assays that detect specific malaria antigens (such as HRP2 and pLDH) in a small blood sample.
- Types: Different RDTs target various antigens, allowing for the detection of different Plasmodium species.
- Advantages:
- Ease of Use: Simple and can be used in field settings with minimal training.
- Rapid Results: Provides results within 15-20 minutes.
- No Need for Special Equipment: Does not require a microscope or laboratory infrastructure.
- Limitations:
- Sensitivity and Specificity: Variable performance depending on the brand and quality of the test. False negatives can occur, especially with low parasite densities or in cases of drug resistance.
- Limited Species Differentiation: Some RDTs may not differentiate between all Plasmodium species or may not detect certain species.
- Recent Developments:
- Improved RDTs: Newer RDTs with enhanced sensitivity and specificity, and the ability to detect multiple species and low parasite counts.
- Description:
Polymerase Chain Reaction (PCR)
- Description:
- Method: PCR amplifies and detects specific DNA or RNA sequences of malaria parasites. It is highly sensitive and can identify low levels of parasitemia.
- Types: Conventional PCR, real-time PCR (qPCR), and nested PCR.
- Advantages:
- High Sensitivity: Can detect very low levels of parasites and identify all Plasmodium species.
- Species Identification: Allows for accurate species identification and detection of mixed infections.
- Limitations:
- Complexity: Requires specialized equipment and trained personnel.
- Cost and Time: More expensive and time-consuming compared to microscopy and RDTs, making it less suitable for field settings.
- Recent Developments:
- Point-of-Care PCR: Efforts are underway to develop portable PCR devices for use in remote or low-resource settings.
- Description:
Loop-Mediated Isothermal Amplification (LAMP)
- Description:
- Method: LAMP is a nucleic acid amplification technique that uses specific primers and enzymes to amplify Plasmodium DNA at a constant temperature.
- Advantages: Similar to PCR but simpler and does not require complex equipment.
- Advantages:
- High Sensitivity and Specificity: Effective in detecting low levels of parasitemia.
- Ease of Use: Can be performed with minimal training and equipment.
- Limitations:
- Less Established: LAMP is less widely used than PCR, and protocols may vary.
- Recent Developments:
- Field Adaptation: LAMP is being adapted for use in field settings, providing a promising alternative to PCR in low-resource areas.
- Description:
Serological Tests
- Description:
- Method: Serological tests detect antibodies or antigens produced in response to malaria infection.
- Types: Enzyme-linked immunosorbent assay (ELISA) and indirect fluorescent antibody tests (IFAT).
- Advantages:
- Detects Previous Infections: Useful for epidemiological studies and detecting past infections.
- Complementary to Other Tests: Can be used in conjunction with other diagnostic methods for comprehensive diagnosis.
- Limitations:
- Not Useful for Acute Diagnosis: Less effective for diagnosing active infections, as antibodies may take time to develop.
- Recent Developments:
- Rapid Serological Tests: New serological tests are being developed to provide quicker results and improved accuracy.
- Description:
Treatment
Historical perspective
Before modern antimalarials, malaria was treated with various traditional remedies. One of the earliest and most effective treatments was the bark of the Cinchona tree, which contains quinine. Indigenous people in South America used this bark to treat fevers, a practice later adopted by Europeans in the 17th century. Quinine became the first successful treatment for malaria and remained the primary antimalarial until the 20th century. Other historical remedies included the use of the Chinese herb Artemisia annua, which contains artemisinin, a compound now central to modern antimalarial therapies. Additionally, ancient Egyptians and Greeks used herbal concoctions, and the Romans recognized the importance of draining swamps to reduce mosquito populations.
Pre-Quinine Era
- Ancient Remedies:
- Egyptian and Greek Medicine: Ancient Egyptian texts suggest treatments for malaria-like symptoms, using substances like honey and various plant extracts. Greek physicians such as Hippocrates described symptoms that resemble malaria but lacked effective treatments.
- Traditional Chinese Medicine: Ancient Chinese texts, including those by Shen Nong, document the use of herbal remedies for treating fever and other symptoms related to malaria. These practices laid the groundwork for later discoveries.
Quinine and Early 19th Century
Cinchona Bark in Europe:
- Introduction and Early Use: Jesuit missionaries introduced quinine to Europe in the 17th century. Initially used to treat fevers, it soon became recognized for its antimalarial properties.
- Medical Adoption: By the early 19th century, quinine was widely adopted as the primary treatment for malaria. Its use became integral in European colonial expansion and the establishment of tropical plantations.
Advancements in Quinine Production:
- Extraction and Purification: Early chemists like Joseph Pelletier and Joseph Bienaimé Caventou improved the extraction and purification of quinine in the 1820s. This standardization increased its efficacy and accessibility.
Early 20th Century
Synthetic Drug Development:
- Chloroquine: Developed in the 1930s, chloroquine became a revolutionary drug in malaria treatment. Its introduction followed the discovery of synthetic antimalarials, which aimed to address the limitations of quinine.
- Quinine Alternatives: During World War II, quinine shortages led to increased use of chloroquine. Chloroquine’s effectiveness in treating malaria led to its widespread use and incorporation into malaria control programs.
Primaquine and Comprehensive Treatment:
- Introduction: Primaquine was introduced in the 1940s to address the liver stages of malaria caused by Plasmodium vivax. Its use was crucial for preventing relapses and was a significant advancement in malaria treatment.
Mid-20th Century to Late 20th Century
Resistance Development:
- Chloroquine Resistance: By the 1950s and 60s, resistance to chloroquine emerged, particularly in Southeast Asia. This resistance spread to Africa, prompting urgent research into new treatments.
- Mefloquine: Developed in the 1970s, mefloquine provided a new option for treating chloroquine-resistant malaria. Its introduction was a key development in managing drug-resistant strains.
Combination Therapies:
- Combination Therapies: In response to resistance issues, researchers explored combining different antimalarial drugs to enhance efficacy and reduce the risk of resistance. This approach became more sophisticated with the advent of ACTs.
Current Landscape
Modern-day malaria treatments focus primarily on the use of antimalarial drugs, with Artemisinin-based Combination Therapies (ACTs) being the cornerstone of treatment for Plasmodium falciparum malaria, the most severe form of the disease. The shift to ACTs as the first-line treatment emerged due to the increasing prevalence of drug-resistant malaria parasite strains.
Artemisinin-Based Combination Therapies (ACTs)
Artemisinin-based Combination Therapies (ACTs) are the cornerstone of modern malaria treatment, particularly for Plasmodium falciparum infections. They consist of an artemisinin derivative combined with a partner drug that has a different mode of action. This combination approach is used to enhance treatment efficacy, reduce the duration of treatment, and prevent the development of drug resistance. Artemisinin and its derivatives, such as artemether, artesunate, and dihydroartemisinin, act rapidly against the malaria parasite. These compounds are derived from Artemisia annua and are highly effective in clearing parasites from the bloodstream. The molecular mechanism of action of artemisinin and its derivatives involves the generation of free radicals upon activation by heme, leading to widespread damage to the parasite’s cellular components. To combat resistance, artemisinin is combined with other antimalarial drugs with different mechanisms of action, ensuring a synergistic effect. This combination not only improves efficacy but also helps prevent the development of drug resistance. The most commonly used partner drugs include lumefantrine, mefloquine, amodiaquine, and piperaquine. The World Health Organization (WHO) recommends ACTs as the first-line treatment for uncomplicated P. falciparum malaria due to their effectiveness, fast action, and role in reducing transmission. The adoption of ACTs has significantly improved malaria treatment outcomes globally, although challenges like drug resistance and accessibility in remote areas persist.
- Common ACT Regimens
Artemether-Lumefantrine (Coartem): One of the most widely used ACTs, typically administered as a six-dose regimen over three days. It is effective against drug-resistant P. falciparum.
Artesunate-Mefloquine: Commonly used in Southeast Asia, where it remains highly effective despite concerns about mefloquine resistance.
Artesunate-Amodiaquine: Often used in Africa and recommended in many national treatment guidelines.
Dihydroartemisinin-Piperaquine: Notable for its longer half-life, which offers the advantage of a simpler dosing regimen (typically once daily for three days).
Artemether-Benflumetol (Lumefantrine): Another formulation of artemether-lumefantrine.
- Usage and Administration
- Treatment Regimen: ACTs are typically administered over a three-day course, depending on the specific combination. The rapid action of artemisinin derivatives reduces the parasite load, while the partner drug’s longer action period ensures complete clearance of the infection.
- Indications: ACTs are primarily indicated for the treatment of uncomplicated P. falciparum malaria. They are also used in some cases for P. vivax malaria, particularly where resistance to chloroquine has developed.
- Dosing Considerations: Dosages are weight-based, and adherence to the full course is crucial for preventing resistance development and ensuring treatment efficacy.
ACTs represent the frontline treatment for malaria, combining rapid-acting artemisinin derivatives with longer-acting partner drugs. They offer high efficacy and help prevent resistance, making them crucial in the global fight against malaria. Continued monitoring for drug resistance, along with research into new therapeutic options, remains essential to sustain their effectiveness.
Atovaquone-Proguanil (Malarone):
Malarone, a combination of atovaquone and proguanil, is a widely used antimalarial medication in modern-day interventions. It is effective for both the prevention and treatment of malaria. Atovaquone disrupts the mitochondrial electron transport chain in the parasite, specifically inhibiting the cytochrome bc1 complex. This action leads to the collapse of mitochondrial membrane potential and disruption of ATP synthesis, ultimately killing the parasite. Proguanil is a prodrug that is converted in the body to its active form, cycloguanil. Cycloguanil inhibits dihydrofolate reductase (DHFR), an enzyme crucial for folate synthesis in the parasite, impairing DNA synthesis and cell division. The combination of atovaquone and proguanil works synergistically to effectively clear parasitemia.
Prophylaxis (Prevention):
- Malarone is widely used as a prophylactic medication for travelers to areas with P. falciparum malaria, especially where chloroquine-resistant strains are prevalent. It is highly effective and generally well-tolerated, with the added convenience of a short dosing regimen: it is taken daily, starting 1-2 days before entering an endemic area, continued throughout the stay, and for 7 days after leaving the area. This shorter post-exposure period is a notable advantage compared to other prophylactic medications like mefloquine or doxycycline, which require longer periods of post-travel dosing.
Treatment of Acute Malaria:
- Malarone is also used for the treatment of uncomplicated malaria. Its efficacy against chloroquine-resistant and multidrug-resistant strains of P. falciparum makes it a valuable option, especially in regions where these resistances are common. The typical treatment course is three days, which is shorter than many other antimalarials, improving compliance.
Malarone remains an important tool in the prevention and treatment of malaria, particularly for travelers and in regions with drug-resistant P. falciparum. Its effectiveness, tolerability, and user-friendly dosing schedule make it a preferred choice for many, despite considerations around cost and potential resistance. Its role in modern antimalarial interventions continues to be significant, contributing to the global effort to control and eventually eradicate malaria.
Quinine
Quinine has a long history of use in the treatment of malaria. Although its use has declined with the advent of more effective and better-tolerated medications, itstill plays a crucial role in certain clinical situations, particularly for severe malaria or when other treatments are not available or suitable. Quinine works by interfering with the parasite’s ability to metabolize hemoglobin, leading to the accumulation of toxic heme within the parasite’s food vacuole, which ultimately kills the parasite.
Treatment of Severe Malaria:
- Quinine is primarily used to treat severe Plasmodium falciparum malaria, especially in cases where artesunate, the preferred treatment, is not available. It is effective against blood-stage parasites, including drug-resistant strains. Quinine is administered intravenously in a hospital setting for severe cases. The typical dosage involves an initial loading dose followed by maintenance doses every 8 hours. Once the patient can tolerate oral medication, quinine may be transitioned to oral therapy to complete the treatment course.
Oral Quinine for Uncomplicated Malaria:
- In some regions, oral quinine may still be used for uncomplicated malaria, often in combination with another drug, such as doxycycline or clindamycin, to ensure efficacy and prevent resistance.
Side Effects and Monitoring:
- Quinine can cause several side effects, including cinchonism (characterized by symptoms such as tinnitus, headache, nausea, and visual disturbances), hypoglycemia, and, rarely, severe hypersensitivity reactions. Monitoring is essential, especially when administered intravenously.
Quinine, though largely superseded by newer treatments, remains an important drug in the global fight against malaria, particularly in severe cases or where newer therapies are unavailable. Its use requires careful medical supervision due to potential side effects and the need for specific dosing and monitoring protocols. Its historical significance and continued utility in certain scenarios underscore its role in the history and ongoing management of malaria.
Funding for Malaria Combat and Research
The history of funding for malaria control and research reflects a complex interplay of scientific advancements, public health priorities, global economic conditions, and geopolitical factors. This timeline provides an overview of key phases and milestones in the history of malaria funding.
Early 20th Century: Initial Public Health Efforts and Colonial Concerns
- 1900s: Following the discovery of the role of mosquitoes in malaria transmission by Sir Ronald Ross, colonial powers began funding efforts to control the disease in tropical colonies. These efforts included draining swamps, distributing quinine, and implementing basic public health measures.
- 1920s-1930s: The League of Nations and other international bodies began advocating for malaria control. Funding was still limited and primarily came from colonial administrations and missionary organizations.
Mid-20th Century: The Global Malaria Eradication Campaign
- 1940s-1950s: Post-World War II, the advent of DDT and other insecticides, along with the discovery of new antimalarial drugs, spurred significant international investment in malaria control. The United States and other nations, along with organizations like the World Health Organization (WHO), funded large-scale eradication efforts.
- 1955-1969: The WHO launched the Global Malaria Eradication Programme (GMEP), funded by national governments and international agencies. Despite substantial investment, the program was not universally successful and was eventually abandoned in 1969 due to logistical challenges, drug resistance, and funding shortfalls.
Late 20th Century: Shift to Control and Resurgence
- 1970s-1980s: As eradication efforts waned, funding declined, and malaria resurged in many areas. Control efforts were more localized, with limited funding primarily from national governments and bilateral aid. The emphasis shifted towards control rather than eradication, focusing on vector control, drug distribution, and public health campaigns.
- 1990s: A renewed interest in malaria was spurred by the recognition of drug-resistant strains and the increased burden of the disease. This period saw the rise of international NGOs and private foundations (e.g., the Bill & Melinda Gates Foundation) becoming key players in funding malaria research and control.
Early 21st Century: Global Initiatives and Increased Investment
- 2000: The Roll Back Malaria Partnership was established, bringing together governments, NGOs, and international agencies to coordinate efforts and funding. The Global Fund to Fight AIDS, Tuberculosis, and Malaria was also established, significantly increasing financial resources for malaria control and prevention.
- 2000s: The President’s Malaria Initiative (PMI) was launched by the United States, providing significant funding for malaria control in Africa. Other countries and organizations, including the WHO and the World Bank, also increased their financial commitments.
- Bill & Melinda Gates Foundation: Became a major donor, funding research for new treatments, vaccines, and control strategies. The foundation’s significant investments helped catalyze further funding and interest in malaria research.
Recent Developments: Research Advancements and Global Health Funding
- 2010s: Global funding for malaria continued to increase, peaking at around $3 billion annually. Funding focused on implementing control measures (like bed nets and ACTs), research into drug resistance, and vaccine development. The development of the RTS,S/AS01 (Mosquirix) vaccine marked a significant achievement, receiving substantial funding for research and deployment.
- COVID-19 Pandemic Impact: The pandemic disrupted malaria control efforts and funding, with resources being diverted to the COVID-19 response. However, global health organizations emphasized maintaining malaria funding to prevent setbacks.
- 2020s: The introduction and rollout of new tools, such as the RTS,S vaccine, and continued research into novel interventions, including monoclonal antibodies and gene drive technologies for mosquito control. Funding remains robust, with increasing emphasis on sustainable financing and integration with broader health systems.
Funding for malaria remains critical, with international donors, national governments, and private foundations playing key roles. The Global Fund, PMI, and the Gates Foundation continue to be major contributors. There is a growing emphasis on domestic funding and innovative financing mechanisms to ensure long-term sustainability.
Challenges
Addressing both scientific and non-scientific challenges is crucial for the effective control and eventual eradication of malaria. A multidisciplinary approach involving medical research, public health policy, education, and community engagement is necessary to overcome these barriers and make substantial progress in the fight against malaria.
Scientific Challenges
Drug Resistance:
- One of the most significant scientific challenges in the fight against malaria is the emergence and spread of drug-resistant strains of the Plasmodium parasite, particularly Plasmodium falciparum. Resistance to chloroquine, once the mainstay of malaria treatment, emerged in the 1950s and 1960s. More recently, resistance to artemisinin-based combination therapies (ACTs), the current frontline treatment, has been reported in Southeast Asia. This resistance threatens the efficacy of existing treatment regimens and necessitates the development of new antimalarial drugs.
Insecticide Resistance:
- The effectiveness of vector control methods, such as insecticide-treated nets (ITNs) and indoor residual spraying (IRS), is compromised by the increasing resistance of mosquito vectors to commonly used insecticides. Resistance to pyrethroids, the most widely used class of insecticides in ITNs, poses a significant challenge, as it reduces the efficacy of this critical preventive measure.
Complexity of the Parasite’s Life Cycle:
- The Plasmodium parasite’s complex life cycle, involving both human and mosquito hosts and multiple developmental stages, complicates efforts to control and eliminate the disease. The presence of dormant liver stages (hypnozoites) in P. vivax and P. ovale infections can cause relapses, making eradication efforts more challenging.
Limited Vaccine Efficacy:
- While the RTS,S/AS01 (Mosquirix) and R21/Matrix-M vaccines represent a significant scientific achievement, they provide only partial protection and require multiple doses for optimal efficacy. The development of a highly effective, long-lasting malaria vaccine remains a critical unmet need in the global effort to combat malaria.
Diagnostic Challenges:
- Accurate diagnosis is essential for effective treatment and management of malaria. However, the sensitivity of rapid diagnostic tests (RDTs) can vary, particularly in cases of low parasitemia or mixed infections. Additionally, asymptomatic carriers who do not seek treatment can continue to transmit the disease.
Non-Scientific Challenges
Socioeconomic Factors:
- Malaria disproportionately affects low-income countries and regions, where healthcare infrastructure, access to treatment, and preventive measures are often inadequate. Poverty, lack of education, and limited access to healthcare services hinder effective malaria control and contribute to higher disease burden and mortality rates.
Political and Economic Instability:
- Political instability, conflict, and economic crises can disrupt malaria control programs, leading to lapses in prevention, diagnosis, and treatment efforts. In regions experiencing conflict or displacement, healthcare systems are often overwhelmed, and access to essential services, including malaria treatment, is severely limited.
Healthcare Infrastructure:
- In many endemic areas, the healthcare infrastructure is insufficient to support comprehensive malaria control measures. This includes limited availability of diagnostic tools, medications, and trained healthcare professionals. In remote and rural areas, logistical challenges further complicate the delivery of healthcare services.
Cultural and Behavioral Factors:
- Cultural beliefs and practices can influence the acceptance and utilization of malaria control measures. For example, misconceptions about the causes of malaria, reluctance to use insecticide-treated nets, and reliance on traditional healers instead of formal medical treatment can all hinder effective disease management.
Funding and Resource Allocation:
- Sustained funding is critical for maintaining and scaling up malaria control efforts. However, fluctuations in international funding and donor fatigue can lead to resource shortages, undermining progress. Moreover, the allocation of limited resources often needs to balance between malaria and other public health priorities, complicating efforts to secure adequate funding.
Climate Change and Environmental Factors:
- Climate change can affect the transmission dynamics of malaria by altering the distribution and breeding patterns of mosquito vectors. Changes in temperature, rainfall, and humidity can expand the geographical range of malaria transmission to previously non-endemic areas, complicating control efforts.
Curiosities
“Quinine Trees” and the Origins of Antimalarial Drugs
- The discovery of quinine, the first effective antimalarial treatment, has a fascinating history rooted in indigenous practices. The Cinchona tree, native to the Andes in South America, produces bark that was traditionally used by the Quechua people to treat shivering. The active component, quinine, was extracted from the bark and was found to have antipyretic (fever-reducing) properties. The introduction of quinine to Europe is attributed to Jesuit missionaries in the 17th century, earning it the name “Jesuit’s Bark.” It became the first chemical compound used to treat malaria, revolutionizing medicine and significantly impacting European colonization efforts in malaria-endemic regions. The British colonial administration in India and Africa relied heavily on quinine to protect troops and settlers from malaria, which was a major cause of mortality.
Malaria as a “Curse of the Pharaohs”
- Malaria’s presence in ancient civilizations is evidenced by the detection of malaria DNA in Egyptian mummies. Historical texts and artworks also suggest that malaria was prevalent in ancient Egypt. The disease may have contributed to the decline of the Egyptian empire, as repeated outbreaks could have weakened the population and military forces. The ancient Greek and Roman civilizations also documented fevers that could have been caused by malaria. Hippocrates wrote about periodic fevers, distinguishing between tertian (every third day) and quartan (every fourth day) fevers, which correspond to the fever patterns of Plasmodium vivax and Plasmodium malariae, respectively.
The Connection Between Malaria and the Great Sphinx of Giza
- It is speculated that malaria may have contributed to the abandonment and burial of the Great Sphinx of Giza. During periods when the Nile’s inundation created stagnant pools of water, mosquito populations would surge, increasing malaria transmission. This health hazard, along with other factors, may have led to reduced habitation and maintenance of the area, contributing to the Sphinx being covered by sand for many centuries.
Malaria’s Influence on Human Genetics
Malaria has exerted significant evolutionary pressure on human populations, leading to the selection of genetic traits that confer some protection against the disease.- Sickle cell trait. Sickle cell trait occurs when an individual inherits one normal hemoglobin gene (HbA) and one sickle hemoglobin gene (HbS) from their parents. This genetic condition results in the production of both normal hemoglobin and some sickle-shaped red blood cells. Individuals who carry one copy of the sickle cell gene (heterozygotes) are partially protected against P. falciparum malaria because the altered shape and decreased lifespan of sickle cells create an inhospitable environment for the malaria parasite, inhibiting its growth and multiplication, and infected sickle cells are more readily recognized and destroyed by the immune system or filtered out by the spleen.
- Thalassemias: Individuals with thalassemias, particularly alpha-thalassemia, have been observed to have some protection against severe forms of malaria. Thalassemia causes structural and functional changes in red blood cells, making them less less efficient at supporting parasite growth and replication.
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency: This condition, which can cause hemolytic anemia, is associated with some level of protection against severe malaria. The oxidative stress resulting from G6PD deficiency can hinder the survival and replication of malaria parasites within red blood cells, while G6PD-deficient red blood cells are more susceptible to oxidative damage, including from the malaria parasites themselves, which can lead to increased destruction of these infected cells.
The Etymology of “Malaria”
- The term “malaria” originates from the Italian “mala aria,” meaning “bad air.” This reflects the ancient belief that the disease was caused by the miasma, or harmful vapors, emanating from swamps and marshes. Before the discovery of the actual cause of malaria, it was commonly thought that the disease spread through unhealthy air, particularly in warm, damp regions. This belief influenced urban planning and sanitation efforts in many societies, as they attempted to combat the “bad air” to prevent disease.
The Papal Conclave of 1623 and Malaria
The papal conclave of 1623, held to elect a successor to Pope Gregory XV, is a notable historical event marked by an outbreak of malaria among the cardinals. The conclave, which took place during the hot summer months in Rome, saw several cardinals and conclave staff members fall ill with what was likely malaria. The disease, then often referred to as “Roman fever,” was prevalent in the area, particularly in the humid and stagnant air around the Vatican.
- Location and Time: The conclave was held in the Vatican, a region known at the time for its marshy surroundings, which were breeding grounds for malaria-carrying mosquitoes.
- Victims: Several cardinals, including the prominent figures who were papal candidates, succumbed to the illness. Cardinal Robert Bellarmine and Cardinal Alessandro Ludovisi (who later became Pope Gregory XV) are among those who suffered from malaria. While Bellarmine survived, other less fortunate cardinals passed away either during or shortly after the conclave.
- Outcome: The conclave ultimately elected Cardinal Maffeo Barberini as Pope Urban VIII, who himself contracted malaria during the proceedings but survived. The deaths and illnesses of many cardinals during this conclave underscored the endemic nature of malaria in Rome at the time and the risk it posed even to the highest ranks of the Catholic Church.
Malaria and the Military
Throughout history, malaria has had a profound impact on military campaigns. For centuries, armies moving through or stationed in malaria-endemic areas faced significant morbidity and mortality from the disease, sometimes more than from actual combat. For example:- The Roman Empire: Malaria is believed to have played a significant role in the decline of the Roman Empire. The disease was rampant in the swampy areas around Rome, particularly in the Pontine Marshes. The constant outbreaks of malaria weakened the population and military forces, contributing to the empire’s vulnerability to external invasions. The Roman infrastructure, including aqueducts and roads, may have inadvertently facilitated the spread of malaria by creating environments conducive to mosquito breeding.
- The American Civil War: Malaria was a significant cause of illness among troops, with both Union and Confederate soldiers suffering high rates of infection.
- World War II: Malaria posed a major threat to Allied forces in the Pacific and African theaters. The widespread use of quinine and later synthetic antimalarials like chloroquine and mefloquine were critical in maintaining troop health and operational effectiveness.
Malaria and the Italian Campaign in World War II
- During World War II, the Italian Campaign faced a severe malaria outbreak. The draining of the Pontine Marshes, initially aimed at controlling malaria, was reversed when German forces flooded the area to create a defensive barrier against the Allies. This flooding led to a resurgence of malaria, severely affecting both soldiers and civilians. The disease became a significant obstacle for the Allies, who struggled to control the spread among troops.
Malaria in the Panama Canal Construction
- The construction of the Panama Canal was heavily affected by malaria and yellow fever, both transmitted by mosquitoes. The high mortality rate among workers due to these diseases nearly halted the project. It was only after the implementation of widespread mosquito control measures, led by Dr. William Gorgas, that the construction could proceed. These measures included draining standing water, using insecticides, and introducing mosquito nets, which significantly reduced the incidence of malaria among workers and allowed the successful completion of the canal.
The Influence of Malaria on Exploration and Colonization
- Malaria has significantly influenced global exploration and colonization. The disease posed a major barrier to European exploration of tropical regions, including parts of Africa, Asia, and the Americas. The high mortality rates among explorers, soldiers, and settlers often deterred colonization efforts. The eventual development and distribution of antimalarials like quinine were crucial in enabling the colonization and exploitation of tropical regions by European powers. Many historical explorers and expeditions were significantly impacted by malaria. For example, David Livingstone, the famous Scottish explorer, and missionary, suffered from malaria multiple times during his travels in Africa. His writings detailed the challenges posed by the disease and contributed to European knowledge of Africa. Similarly, malaria posed a significant risk during early explorations of the Amazon and other tropical regions, often determining the success or failure of these expeditions.
Malaria and the Slave Trade
- Malaria had a significant impact on the transatlantic slave trade. The high prevalence of malaria (and other diseases like yellow fever) in the Caribbean and American South led to high mortality rates among European settlers. African slaves, who often had some degree of immunity to Plasmodium falciparum due to lifelong exposure, were tragically perceived as more suitable for labor in these areas. This perception contributed to the demand for enslaved Africans and influenced the demographic and economic landscapes of the Americas.
Malaria and the British Empire
- Malaria significantly affected British colonial efforts, particularly in Africa and India. The British East India Company, for instance, struggled with high mortality rates among its employees due to malaria. This led to the establishment of medical services and research into the disease. Quinine became a critical tool for maintaining British control in these regions. The British army also issued “quinine tablets” as a standard part of a soldier’s ration.
The “Gin and Tonic” Connection
- The popular gin and tonic drink has its origins in malaria prevention. British colonists in India in the 19th century used quinine, derived from the bark of the cinchona tree, to prevent and treat malaria. Quinine was dissolved in carbonated water to create tonic water, but its bitter taste was unpleasant. To make it more palatable, British officers added gin, leading to the creation of the gin and tonic. Although modern tonic water contains much less quinine, the drink remains a popular reminder of its medicinal origins.
The Use of Malaria as a Therapeutic Tool (Malarial Therapy)
- In the early 20th century, before the advent of antibiotics, malaria was used as a treatment for syphilis in a method known as malariotherapy. The idea, introduced by Austrian psychiatrist Julius Wagner-Jauregg, was to induce high fevers through malaria infection, which could kill the syphilis bacteria. The fever from malaria would be induced and then treated with quinine. Wagner-Jauregg received the Nobel Prize in Physiology or Medicine in 1927 for this controversial treatment, which was used until the discovery of penicillin.
Nobel Prize Connections
Several Nobel Prizes have been awarded for research related to malaria, highlighting the significance of scientific discoveries in understanding and combating this disease:
- Charles Louis Alphonse Laveran: Awarded the Nobel Prize in Physiology or Medicine in 1907 for his discovery of the malaria parasite, marking the first time a protozoan was identified as causing disease in humans.
- Ronald Ross: Received the Nobel Prize in 1902 for demonstrating that malaria is transmitted by mosquitoes, specifically the Anopheles species, establishing the vector-borne nature of the disease.
- Paul Hermann Müller: Awarded the Nobel Prize in 1948 for his discovery of the insecticidal properties of DDT, which became a crucial tool in controlling mosquito populations and thus malaria.
- Youyou Tu: Won the Nobel Prize in 2015 for her discovery of artemisinin, a novel and highly effective antimalarial drug derived from the plant
Artemisia annua.
Malaria’s Influence on Literature and Art
- Malaria has been depicted in literature and art throughout history. For example, it is mentioned in ancient Greek texts, including works by Homer and Hippocrates. The disease is also referenced in Shakespeare’s plays, such as “Othello” and “Antony and Cleopatra.” Malaria’s impact on society, including its symptoms and the suffering it causes, has inspired numerous artistic and literary portrayals, reflecting its pervasive influence on human culture.
Malaria and Literary Figures
- Several notable literary figures have had personal experiences with malaria, which influenced their work. For example, Ernest Hemingway contracted malaria during his time in East Africa and later wrote about the disease in his short stories. Graham Greene also suffered from malaria, which influenced his depiction of tropical diseases and environments in novels like “The Heart of the Matter.” These personal encounters with the disease often provided a realistic and poignant portrayal of malaria’s impact on individuals.
Malaria and the Moon Landing
- During the Apollo 11 moon landing in 1969, the U.S. was simultaneously dealing with an outbreak of malaria in Vietnam, where American soldiers were stationed. The malaria issue was so significant that it garnered attention even as the world was focused on the historic moon landing. The military’s focus on managing malaria among troops demonstrated the disease’s impact, even amid groundbreaking achievements like space exploration.
Malaria Research Using Space Technology
- NASA and other space agencies have used satellite technology to monitor and predict malaria outbreaks. Satellites can track environmental conditions such as temperature, humidity, and rainfall, which affect mosquito breeding and malaria transmission. By analyzing this data, scientists can develop early warning systems and targeted interventions to control malaria in vulnerable regions. This innovative approach combines space technology with public health efforts to combat the disease more effectively.
The “Malaria Hotspot” of Southeast Asia
- The Greater Mekong Subregion, which includes countries like Myanmar, Thailand, Laos, Cambodia, and Vietnam, is known as a “malaria hotspot” due to its history of multidrug-resistant Plasmodium falciparum. The region has been a focal point for malaria research and control efforts, especially concerning artemisinin resistance. This resistance poses a significant threat to global malaria control, as artemisinin-based therapies are a cornerstone of treatment.
The Impact of Climate Change on Malaria
- Climate change poses a significant threat to malaria control efforts, as changes in temperature and precipitation patterns can expand the habitats of malaria-carrying mosquitoes. Warmer temperatures can accelerate the life cycle of mosquitoes and the development of malaria parasites within them, potentially increasing transmission rates. Regions that were previously too cool for mosquitoes to thrive may become suitable habitats, leading to a geographic spread of malaria. Increased rainfall can create more breeding sites for mosquitoes, while drought can force populations to concentrate around water sources, increasing transmission risk. Conversely, some areas may experience a reduction in malaria transmission due to conditions becoming unsuitable for mosquito survival.
Conclusion
The term “scourge of humanity” in relation to malaria captures the disease’s extensive historical impact, its severe health and socioeconomic consequences, and the ongoing challenges it presents. Malaria continues to be a significant global health challenge, affecting millions of people annually and causing considerable morbidity and mortality. While substantial progress has been made in understanding the disease, improving diagnostics and treatment, and developing control measures, significant challenges remain. Ongoing research, international collaboration, and sustained commitment are essential to overcoming these challenges and ultimately achieving the goal of malaria elimination and eradication.
Links
Malaria Site – http://www.malariasite.com/
Malaria.com – http://www.malaria.com/
World Health Organization – http://www.who.int/topics/malaria/en/
Center for Disease Control – http://www.cdc.gov/MALARIA/
The Global Fund – https://www.theglobalfund.org/en/malaria/
National Institutes of Health – http://health.nih.gov/topic/Malaria
National Institute of Allergy and Infectious Diseases – http://www.niaid.nih.gov/topics/malaria/Pages/default.aspx
National Health Services – http://www.nhs.uk/Conditions/Malaria/Pages/Introduction.aspx
Bill & Melinda Gates Foundation – http://www.gatesfoundation.org/topics/pages/malaria.aspx
Roll Back Malaria Partnership to end Malaria – http://www.rbm.who.int/
Malaria No More – https://www.malarianomore.org/
Malaria Vaccine Initiative – http://www.malariavaccine.org/
Medicines for Malaria Venture – http://www.mmv.org/
PlasmoDB – https://plasmodb.org/plasmo/app
BEI Resources – https://www.beiresources.org/
The Life Cycles of Plasmodium Parasites and Anopheles Mosquitoes
Plasmodium life cyle
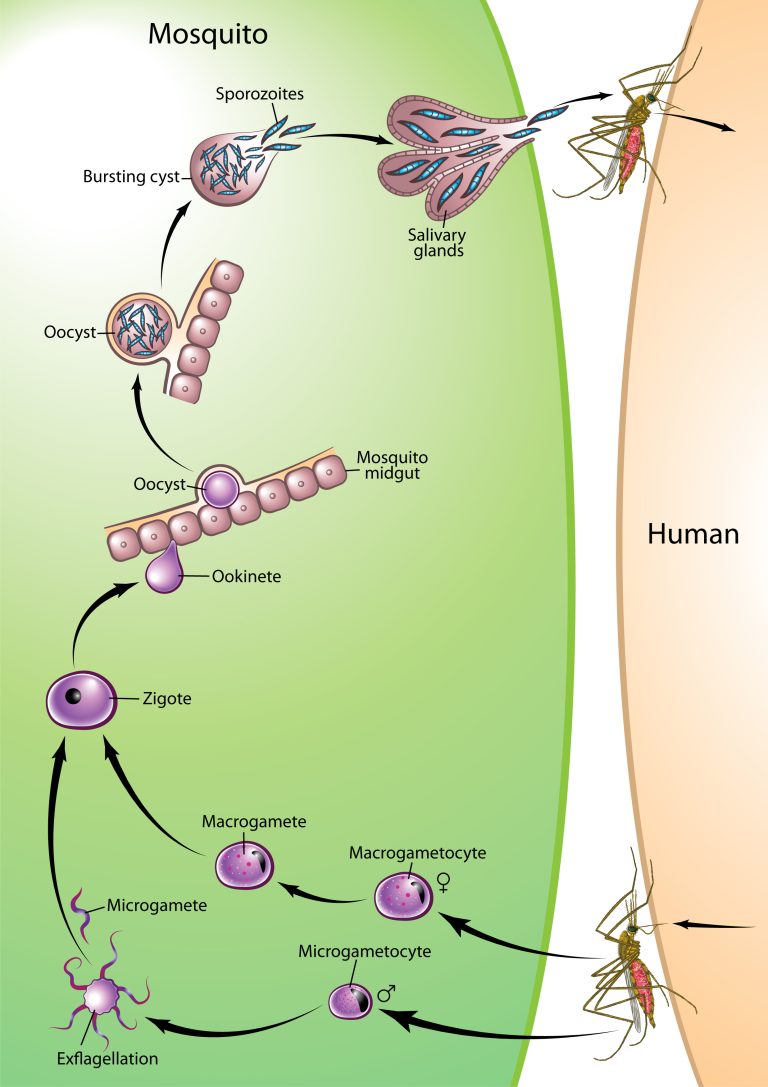
Infection by malaria parasites is initiated when Plasmodium sporozoites enter the mammalian host through the bite of an infected female Anopheles mosquito. Sporozoites deposited under the skin during a blood meal migrate to the liver, where traverse a few hepatocytes and eventually productively invade one, with formation of a parasitophorous vacuole. Inside this vacuole, the parasites replicate extensively and develop into merozoites, which are eventually released into the bloodstream. Each merozoite will invade an erythrocyte, initiating a replication cycle that ends with the release of new merozoites from the mature infected erythrocyte (schizont), which go on to infect other erythrocytes. Malaria- associated pathology only occurs during the blood stage of infection. The Plasmodium life cycle continues when some parasite blood stages develop into the sexual parasite stages, the male and female gametocytes, which can be taken up by mosquitoes during blood meals. Gametocytes undergo fertilization and maturation in the mosquito midgut, forming an infective ookinete form that migrates through the mosquito midgut into the hemocele, developing into the oocyst, in which sporozoites are formed. When fully matured, the oocysts burst and release sporozoites, which migrate into the mosquito’s salivary glands, ready for the next transmission step.
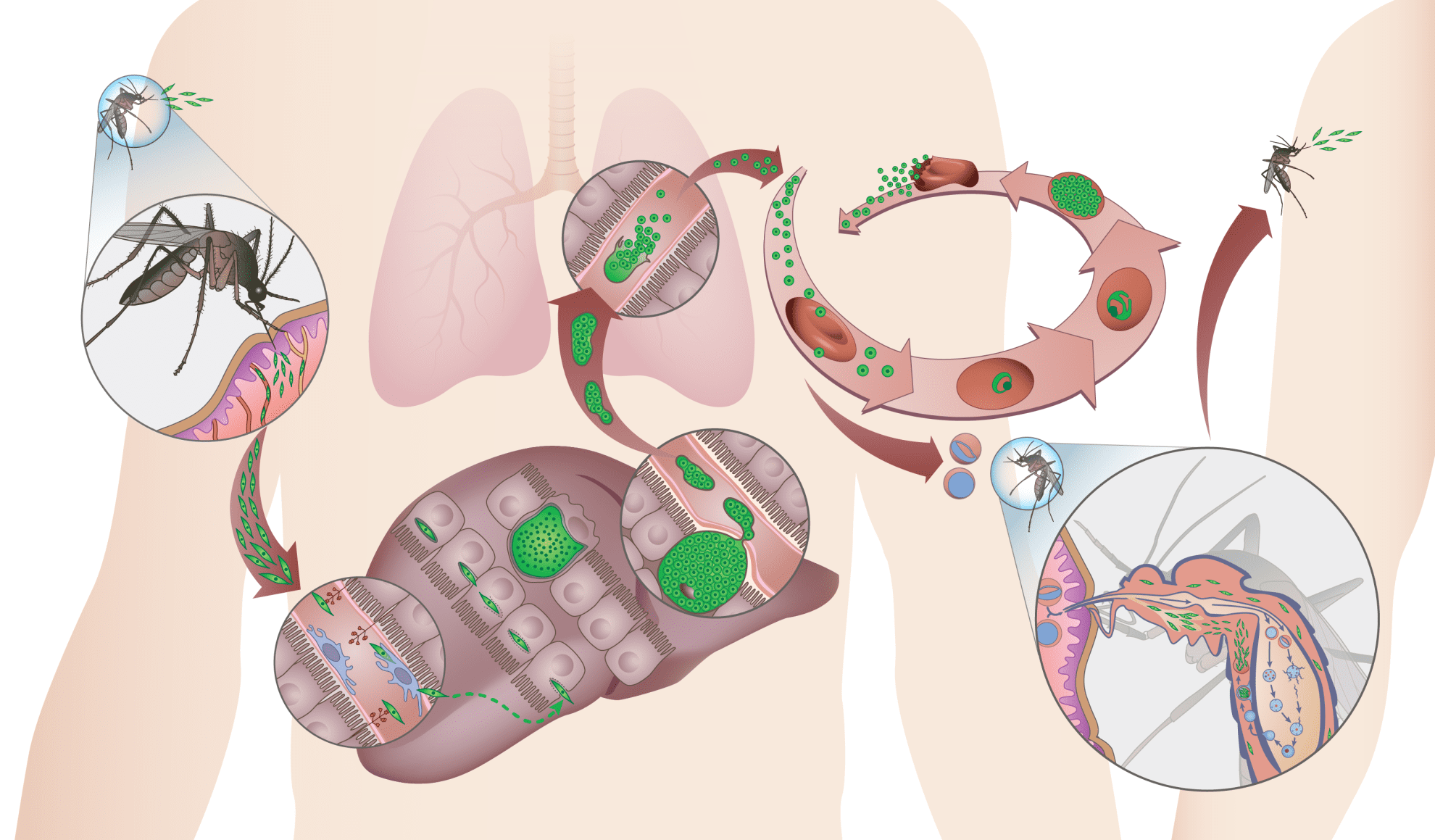
Plasmodium sporogonic cycle
Videos
The life cycle of Plasmodium parasites
Mosquito infected with fluorescent Plasmodium
Plasmodium-infected mosquito salivary gland
Plasmodium sporozoites
Anopheles life cycle
Anopheles mosquitoes go through four separate and distinct stages of its life cycle which are as follows: egg, larva, pupa, and adult. Each of these stages can be easily recognized by their special appearance. Female Anopheles mosquitoes lay their eggs singly on the water. Larvae live in the water and come to the surface to breathe. They shed their skin four times growing larger after each molting. Anopheles larvae lie parallel to the water surface and feed on micro-organisms and organic matter in the water. On the fourth molt the larva changes into a pupa. Pupae are a resting, non-feeding stage. This is the time the mosquito turns into an adult. It takes about two days before the adult is fully developed. When development is complete, the pupal skin splits and the mosquito emerges as an adult. Adults rest on the surface of the water for a short time to allow themselves to dry and all of their parts to harden. Also, the wings have to spread out and dry properly before they can fly.

Species of Plasmodium Parasites and Anopheles Mosquitoes
Plasmodium parasites
Plasmodium is a genus of parasitic protozoa that causes malaria in various vertebrate hosts. The genus includes numerous species, each adapted to specific hosts, ranging from humans and other mammals to birds, reptiles, and amphibians. Here is a comprehensive list of known Plasmodium species, categorized by their respective vertebrate hosts, including both well-documented and lesser-known species across various vertebrate classes:
Plasmodium Species Infecting Humans
- Plasmodium falciparum
- Plasmodium vivax
- Plasmodium malariae
- Plasmodium ovale:
- P. o. curtisi
- P. o. wallikeri
- Plasmodium knowlesi
Plasmodium Species Infecting Non-Human Primates
- Plasmodium cynomolgi
- Plasmodium inui
- Plasmodium simium
- Plasmodium brasilianum
- Plasmodium coatneyi
- Plasmodium schwetzi
- Plasmodium fieldi
- Plasmodium gonderi
- Plasmodium eylesi
- Plasmodium hylobati
- Plasmodium pitheci
- Plasmodium jefferyi
- Plasmodium girardi
- Plasmodium rodhaini
Plasmodium Species Infecting Rodents
- Plasmodium berghei
- Plasmodium yoelii
- Plasmodium chabaudi
- Plasmodium vinckei:
- P. v. vinckei
- P. v. petteri
- P. v. lentum
- P. v. chabaudi
- Plasmodium atheruri
- Plasmodium anasum
- Plasmodium ratnae
- Plasmodium ratti
- Plasmodium gerbillorum
Plasmodium Species Infecting Birds
- Plasmodium relictum
- Plasmodium gallinaceum
- Plasmodium juxtanucleare
- Plasmodium elongatum
- Plasmodium cathemerium
- Plasmodium lophurae
- Plasmodium circumflexum
- Plasmodium hermani
- Plasmodium ashfordi
- Plasmodium columbae
- Plasmodium nucleophilum
- Plasmodium vaughani
- Plasmodium polare
- Plasmodium homocircumflexum
- Plasmodium unalis
- Plasmodium asotae
- Plasmodium gundersi
Plasmodium Species Infecting Reptiles
- Plasmodium mexicanum
- Plasmodium tropiduri
- Plasmodium agamae
- Plasmodium floridense
- Plasmodium azurophilum
- Plasmodium attenuatum
- Plasmodium foleyi
- Plasmodium giganteum
- Plasmodium gonderi
- Plasmodium karyolytum
- Plasmodium rapondoranum
- Plasmodium sasai
- Plasmodium vastator
- Plasmodium leucocytica
- Plasmodium minasense
- Plasmodium wenyoni
Plasmodium Species Infecting Other Mammals
- Plasmodium ovale (occasional human infections, not typically found in non-human hosts)
- Plasmodium knowlesi (occasionally found in macaques)
- Plasmodium semiovale
- Plasmodium eylesi
- Plasmodium schwetzi
- Plasmodium rodhaini (found in chimpanzees and gorillas)
Anopheles mosquitoes
There are over 460 recognized species within the genus Anopheles. Not all species are equally important in the transmission of malaria; some are major vectors, while others may only occasionally transmit the disease or not at all. Additionally, vector competence can vary widely within species due to genetic, environmental, and ecological factors. This list provides an overview of some key Anopheles species ordered roughly by their significance in malaria transmission.
Major Malaria Vectors
- Anopheles gambiae complex
- Anopheles gambiae s.s.: The primary vector of Plasmodium falciparum in sub-Saharan Africa.
- Anopheles coluzzii: Also a significant vector in Africa.
- Anopheles arabiensis: Widely distributed in Africa, known for its adaptability.
- Anopheles quadriannulatus: Primarily zoophilic, lesser role in malaria transmission.
- Anopheles melas: Coastal regions of West Africa.
- Anopheles merus: Coastal and brackish water habitats.
- Anopheles funestus group
- Anopheles funestus: Major vector in sub-Saharan Africa, particularly in East and Southern Africa.
- Anopheles vaneedeni: Known to transmit malaria, but less significant than A. funestus.
- Anopheles darlingi: The primary malaria vector in the Amazon region of South America.
- Anopheles stephensi: Major vector in urban and peri-urban areas in South Asia and the Middle East.
- Anopheles dirus complex
- Anopheles dirus s.s.: Important vector in Southeast Asia.
- Anopheles baimaii: Found in forested areas of Southeast Asia.
- Anopheles minimus complex
- Anopheles minimus s.s.: Major vector in Southeast Asia.
- Anopheles harrisoni: Another important vector in the same region.
- Anopheles albimanus: Important vector in Central America and the northern parts of South America.
- Anopheles culicifacies: Major vector in the Indian subcontinent.
- Anopheles sundaicus: Important vector in Southeast Asia and parts of the Indian Ocean.
- Anopheles maculatus: Found in Southeast Asia, particularly in hilly and forested regions.
- Anopheles balabacensis: Known vector in Southeast Asia, particularly in forested areas.
- Anopheles farauti: Found in the Southwest Pacific, including Papua New Guinea and the Solomon Islands.
- Anopheles fluviatilis: Important in hilly regions of the Indian subcontinent.
- Anopheles nili: Vector in Central Africa.
- Anopheles pharoensis: Found in Africa and parts of the Middle East; plays a role in malaria transmission in specific locales.
- Anopheles sundaicus: Coastal vector in Southeast Asia.
Minor or Regional Vectors
- Anopheles atroparvus: Historically important in Europe.
- Anopheles sacharovi: Important in Turkey and parts of Europe and Central Asia.
- Anopheles culicifacies: A vector in the Indian subcontinent.
- Anopheles albitarsis: Present in South America.
- Anopheles sergentii: Found in North Africa and the Middle East.
Notable Anopheles Species with Limited or Sporadic Role in Malaria Transmission
- Anopheles plumbeus: Found in Europe; occasionally associated with malaria transmission.
- Anopheles freeborni: Historically significant in North America.
