Menu
PbVac
Index
Concept
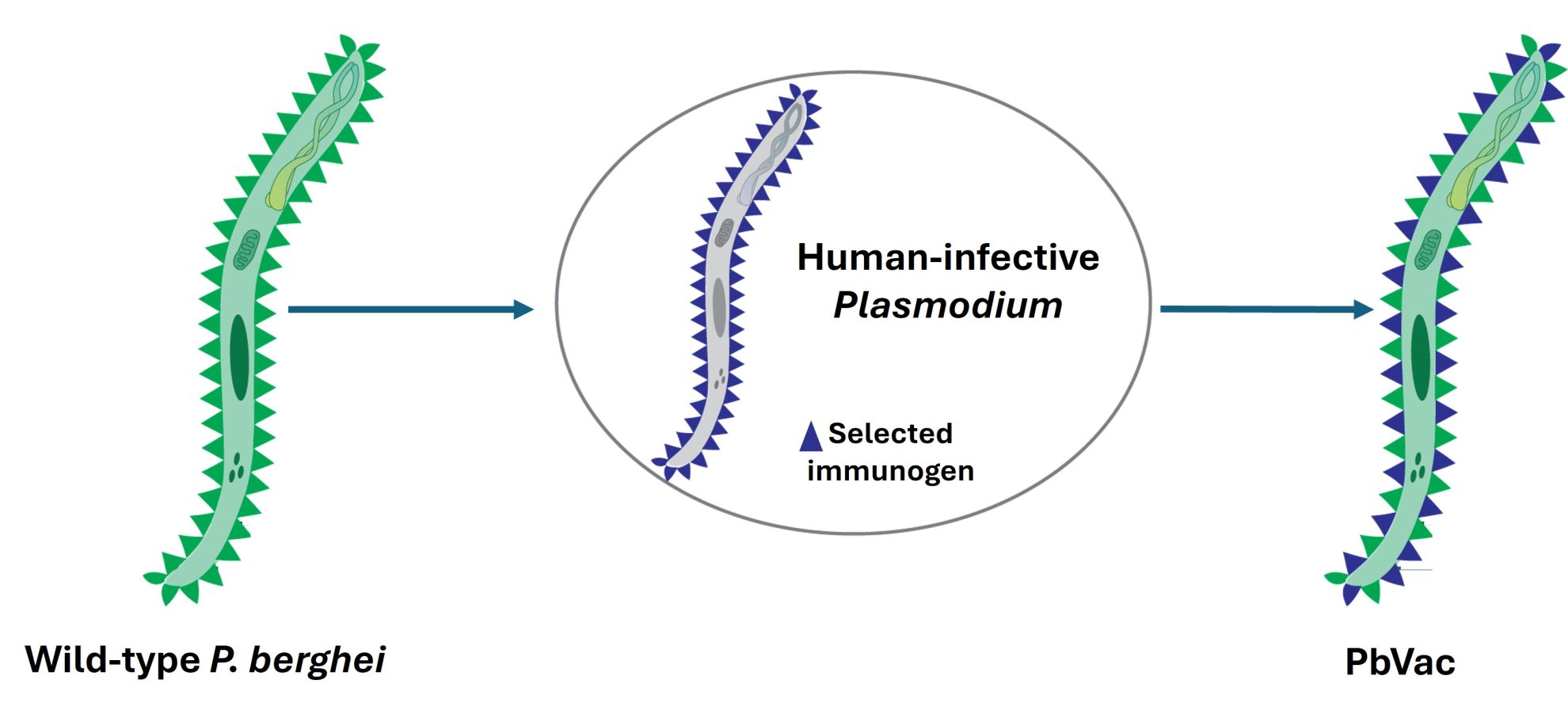
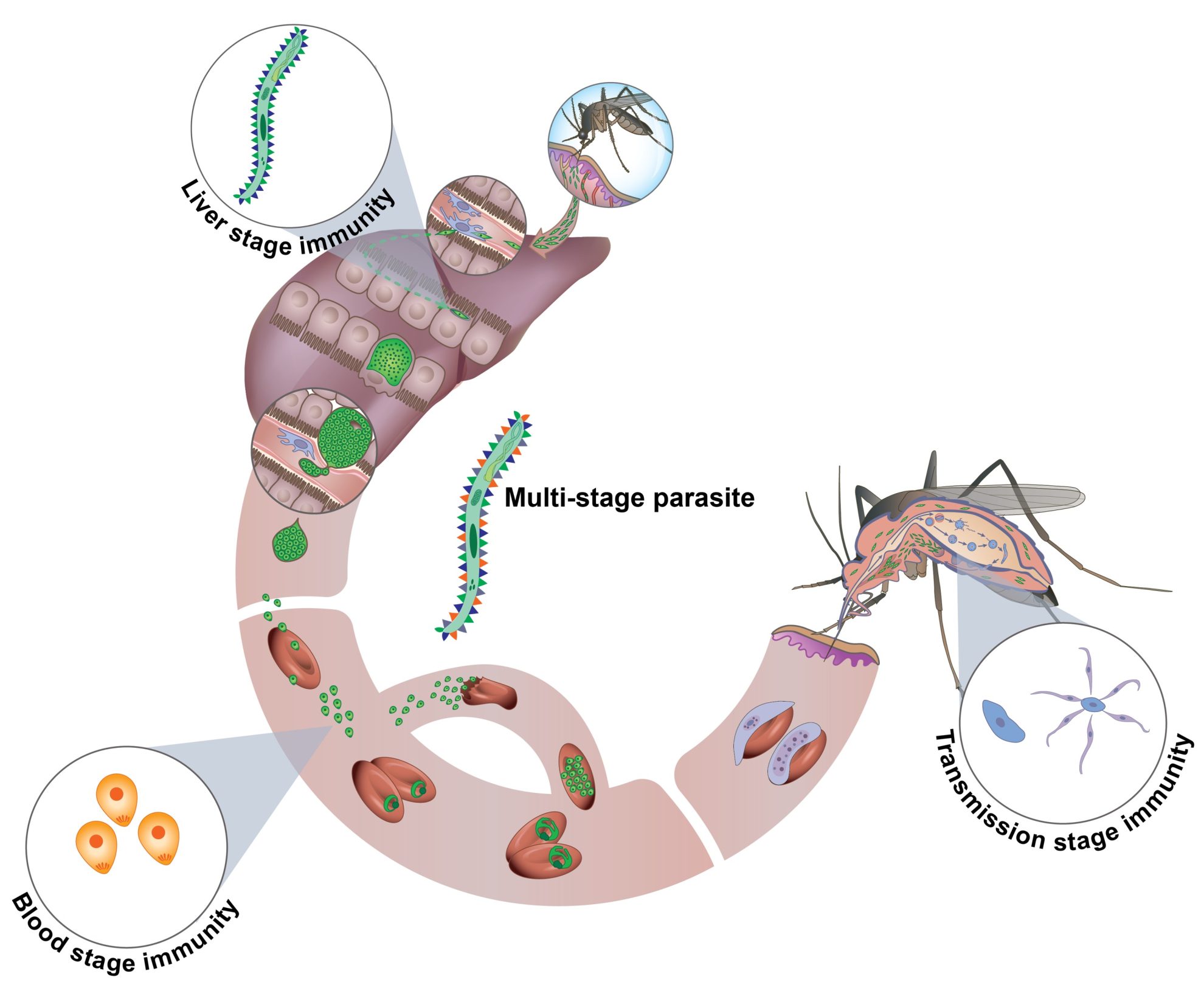
PbVac is a whole-organism platform for vaccination against malaria that employs rodent P. berghei sporozoites, genetically engineered to express selected antigens from their human-infective counterparts, as immunization agents.
Graphics by Raquel Azevedo
Video animation by iMM Communication Office
Overview
PbVac is an experimental malaria vaccine developed as an innovative, whole-organism vaccine platform. Created by modifying the rodent malaria parasite Plasmodium berghei, it is engineered to express a protein (CSP) from the human malaria parasite P. falciparum, responsible for the most severe cases of malaria in humans. This alteration allows PbVac to activate the immune system without causing blood-stage malaria in humans, as the modified parasite can only infect liver cells temporarily and cannot proceed to red blood cells, thus preventing full-blown malaria infection.
The development of PbVac represents a significant step forward in malaria vaccine research, which has traditionally struggled with the parasite’s complex life cycle, especially its liver stages. PbVac has shown strong immunogenicity in preclinical trials by inducing antibodies and immune responses that specifically recognize and attack P. falciparum cells. In the first human trials, completed in 2020, PbVac has proven effective in generating immune responses. Volunteers who received PbVac showed a marked reduction—up to 95%—in the presence of P. falciparum parasites in the liver following exposure. These early results underscore PbVac’s ability to act as a “whole-organism” vaccine, generating comprehensive immunity against liver-stage infection through both antibody production and T-cell activation.
Further work is directed at optimizing the vaccine’s dose and exploring its potential adaptability. This includes the possibility of developing multi-stage vaccines or incorporating antigens for different malaria-causing species like P. vivax, which are difficult to cultivate in laboratory settings. Encouraged by PbVac’s promising results in human trials, the same principle was to create PbViVac, a novel surrogate vaccine against P. vivax, based on genetically modified P. berghei parasites that express the circumsporozoite protein (CSP) of P. vivax. Studies of PbViVac have shown that it induces immune responses specific to P. vivax sporozoites, making it a significant step toward achieving effective protection against P. vivax, which is particularly challenging to target due to its ability to remain dormant in the liver and cause relapses.
PbVac and PbViVac were developed by Dr. Miguel Prudêncio and his team at the Instituto de Medicina Molecular (iMM), now the Gulbenkian Institute for Molecular Medicine (GIMM), in Portugal, as part of a collaborative effort to create innovative malaria vaccination strategies.
Chronology of PbVac development
PbVac
The proof-of-concept of this strategy was established through the development of PbVac, a P. berghei parasite that expresses the P. falciparum CSP antigen on the surface of the parasite’s sporozoites and hepatic stages. With the support of the Bill & Melinda Gates Foundation, the team carried out the pre-clinical characterization and proof-of-concept validation of PbVac, showing that this genetically modified rodent malaria parasite can effectively infect human hepatic cells, a requirement for immunity generation, whilst it is unable to replicate inside human red blood cells, which would otherwise constitute a safety concern. The group further carried out immunization experiments in a variety of animal models, including mice, rabbits and non-human primates, which consistently showed that administration of PbVac elicits both antibody and cellular immune responses that specifically recognize the human P. falciparum parasite (Mendes et al., NPJ Vaccines, 3, 33, 2018).
Upon completion of the pre-clinical characterization of PbVac, and with the support of PATH – Malaria Vaccine Initiative (PATH-MVI), the team carried out an extensive assessment of this vaccine candidate’s safety for human use, towards its evaluation in a clinical setting. This included the creation of a Master Cell Bank of the parasite, the whole-genome sequencing of PbVac, extensive microbiological analyses, a biodistribution study, a drug sensitivity assessment, and a toxicology analysis (Mendes et al., NPJ Vaccines, 3, 54, 2018). This work paved the way for the first-in-man evaluation of PbVac, also supported by PATH-MVI.
In this clinical study, the PbVac vaccine candidate was delivered by mosquito bite, the standard administration method for early-stage clinical assessment of whole-sporozoite malaria vaccines. The clinical evaluation of PbVac included both a Phase 1 safety assessment study and a Phase 2a efficacy trial. The results showed that immunization with this vaccine candidate is (i) safe and well-tolerated; (ii) inhibits a subsequent P. falciparum liver infection by an estimated 95%; and (iii) elicits dose-dependent functional antibody and CD8+ T cell responses against P. falciparum (Reuling et al., Science Translational Medicine, 12, eaay2578, 2020).
PbViVac
Whole-sporozoite vaccines against P. vivax are currently unavailable, largely due to the inability to efficiently produce sporozoites of this human parasite in the laboratory. The P. berghei platform offers the unprecedented possibility of overcoming this limitation, through the engineering of parasite lines that express selected P. vivax antigens. Sporozoites of such transgenic parasites constitute intrinsically safe surrogates for P. vivax whole-sporozoite immunization. With the support of the Portuguese Foundation for Science and Technology (FCT), the Prudêncio lab has constructed a transgenic P. berghei parasite that expresses the P. vivax CSP, termed PbViVac, as a surrogate for a whole-sporozoite P. vivax vaccine. The team has shown that immunization of animal models with PbViVac elicits the production of antibodies against P. vivax CSP that efficiently recognize and bind to P. vivax sporozoites (Moita et al., NPJ Vaccines, 7, 163, 2022).
Ongoing work
The versatility of the P. berghei platform offers the opportunity for insertion of multiple antigens from different stages of either the P. falciparum or P. vivax life cycles, effectively generating multi-stage vaccine candidates against either malaria caused by either of these human parasites. Furthermore, it should be noted that the amount of PbVac inoculated into the volunteers of the clinical study performed was limited by the number of mosquito bites that could be administered per volunteer and was, therefore, necessarily sub-optimal. As such, the observed 95% decrease in the P. falciparum liver burden of the immunized volunteers relative to their non-vaccinated control counterparts, alongside the dose-dependency observed for the immune responses elicited by vaccination, strongly suggest that a higher protective efficacy can be obtained by increasing the dose of vaccine administered. To achieve this, the vaccine candidates must be produced in a vialed, injectable formulation, suitable for inoculation into humans. Methodologies to cryopreserve and vial Plasmodium sporozoites have been developed by the USA-based company Sanaria, Inc. for the production P. falciparum-based whole-sporozoite vaccines intended for human use.
With the support of the “la Caixa” Foundation and the European Commission’s Horizon Europe program, the Prudêncio lab and its collaborators are currently:
- Generating and pre-clinically characterizing an array of transgenic berghei parasites expressing different P. falciparum or P. vivax antigens, in combination with P. falciparum or P. vivax CSP, respectively, in order to create vaccine candidates against P. falciparum and P. vivax malaria with enhanced immunogenicity.
- Working closely with Sanaria, Inc. to purify, vial and cryopreserve berghei-based whole-sporozoite vaccine candidates. At present, cryopreserved versions of both PbVac and PbViVac have been successfully produced under non-GMP conditions and are undergoing pre-clinical validation. Additional candidates will be cryopreserved in the near future, in accordance with the pre-clinical data obtained.
AI-generated podcasts
References
- A.M. Mendes, A. Scholzen, A.K. Mueller, S.M. Khan, R.W. Sauerwein, M. Prudêncio (2017) “Whole Organism Pre-Erythrocytic Vaccines”, In: Rodriguez, A. and Mota M.M. (Eds) Malaria: immune response to infection and vaccination, Springer International Publishing, Cham, Switzerland
- A.M. Mendes, M. Machado, N. Gonçalves-Rosa, I.J. Reuling, L. Foquet, C. Marques, A.M. Salman, A.S.P. Yang, K.A. Moser, A. Dwivedi, C.C. Hermsen, B. Jiménez-Díaz, S. Viera, J.M. Santos, I. Albuquerque, S.N. Bhatia, J. Bial, I. Angulo-Barturen, J.C. Silva, G. Leroux-Roels, C.J. Janse, S.M. Khan, M.M. Mota, R.W. Sauerwein, M. Prudêncio (2018) “A Plasmodium berghei Sporozoite-Based Vaccination Platform Against Human Malaria”, NPJ Vaccines, 3, 33
- A.M. Mendes, I.J. Reuling, C.M. Andrade, T.D. Otto, M. Machado, F. Teixeira, J. Pissarra, N. Gonçalves-Rosa, D. Bonaparte, J. Sinfrónio, M. Sanders, C.J. Janse, S.M. Khan, C.I. Newbold, M. Berriman, C. Lee, Y. Wu, C.F. Ockenhouse, R.W. Sauerwein, M. Prudêncio (2018) “Pre-Clinical Evaluation of a P. berghei-Based Whole-Sporozoite Malaria Vaccine Candidate”, NPJ Vaccines, 3, 54
- I.J. Reuling, A.M. Mendes, G.M. de Jong GM, A. Fabra-García, H. Nunes-Cabaço, G.J. van Gemert, W. Graumans, L.E. Coffeng, S.J. de Vlas, A.S.P. Yang, C.K. Lee, Y. Wu, A.J. Birkett, C.F. Ockenhouse, R. Koelewijn, J.J. van Hellemond, P.J.J. van Genderen, R.W. Sauerwein, M. Prudêncio (2020) “Safety and efficacy of a genetically modified rodent malaria parasite against Plasmodium falciparum malaria: an open-label randomized phase 1/2a trial”, Science Transl. Med.,12, eaay2578.
- D. Moita, T.G. Maia, M. Duarte, C.M. Andrade, I.S. Albuquerque, A. Dwivedi, J.C. Silva, L. González-Céron, C.J. Janse, A.M. Mendes, M. Prudêncio (2022) “A genetically modified Plasmodium berghei parasite as a surrogate for whole-sporozoite vaccination against P. vivax malaria”, NPJ Vaccines, 7, 163, 1-9
